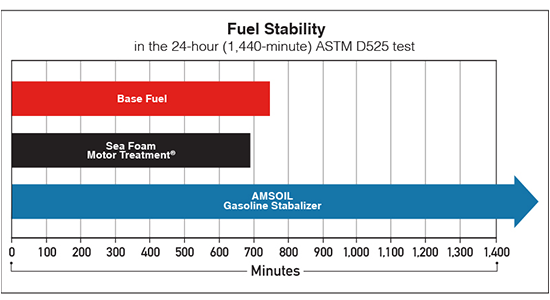
Gasoline is a blend of hydrocarbons and performance-enhancing additives. Over time, this mix can degrade due to chemical and physical changes, leading to poor engine performance and potential damage. The main culprits? Oxidation, evaporation, contamination and polymerization.
Oxidation: The Silent Fuel Killer
Oxidation is the leading cause of gasoline degradation. When hydrocarbons react with oxygen, they form unstable compounds like peroxides. These break down into aldehydes, ketones and acids, which thicken the fuel, reduce its volatility and leave behind sticky deposits. These deposits can clog fuel injectors, carburetors and fuel lines, choking your engine’s performance.
Evaporation: Losing the Lightweights
Gasoline contains light, volatile hydrocarbons like butane and pentane. These evaporate easily, especially in warm environments or poorly sealed containers. As these lighter elements disappear, the fuel becomes harder to ignite, leading to sluggish starts and reduced power.

Contamination: Water and Ethanol Don’t Mix Well
Water can enter fuel tanks through condensation. Ethanol-blended fuels are especially vulnerable because ethanol absorbs water. Above certain concentrations, this can trigger phase separation, where a water layer forms at the bottom of the tank due to its heavier density compared to gasoline. When your engine pulls from this layer instead of pure fuel, it can misfire, stall or experience corrosion.
Polymerization: Gum and Varnish Build-Up
Over time, hydrocarbons and additives in gasoline can link together to form long-chain molecules. This process, accelerated by heat, light and oxygen, creates gum and varnish that coat fuel system components, restricting flow and causing performance issues.









Comments
AMSOIL Market Manager and product expert.
Share: